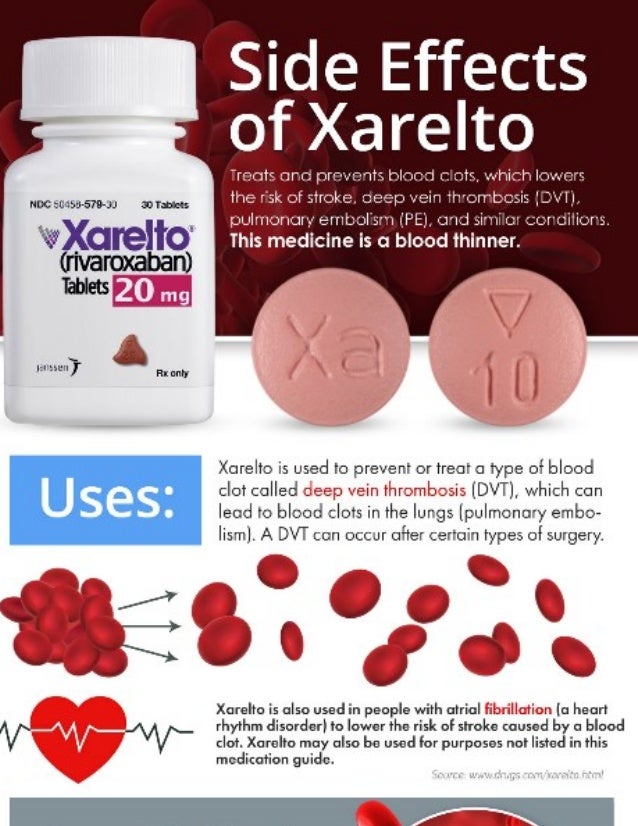
Rivaroxaban (BAY 59-7939) is an oral anticoagulant invented and manufactured by Bayer; in a number of countries it is marketed as Xarelto. In the United States, it is marketed by Janssen Pharmaceutica. It is the first available orally active direct factor Xa inhibitor. Rivaroxaban is well absorbed from the gut and maximum inhibition of factor Xa occurs four hours after a dose. The effects last approximately 8-12 hours, but factor Xa activity does not return to normal within 24 hours so once-daily dosing is possible.

Medical uses
In those with non-valvular atrial fibrillation it appears to be as effective as warfarin in preventing nonhemorrhagic strokes and embolic events. Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin though rivaroxaban is associated with higher rates of bleeding in the gastrointestinal tract.
In September 2008, Health Canada granted marketing authorization for rivaroxaban for the prevention of venous thromboembolism (VTE) in people who have undergone elective total hip replacement or total knee replacement surgery.
In September 2008, the European Commission granted marketing authorization of rivaroxaban for the prevention of venous thromboembolism in adults undergoing elective hip and knee replacement surgery.
On July 1, 2011, the U.S. Food and Drug Administration (FDA) approved rivaroxaban for prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in adults undergoing hip and knee replacement surgery.
On November 4, 2011, the U.S. FDA approved rivaroxaban for stroke prophylaxis in people with non-valvular atrial fibrillation.
What Are The Side Effects Of Xarelto Video
Adverse effects
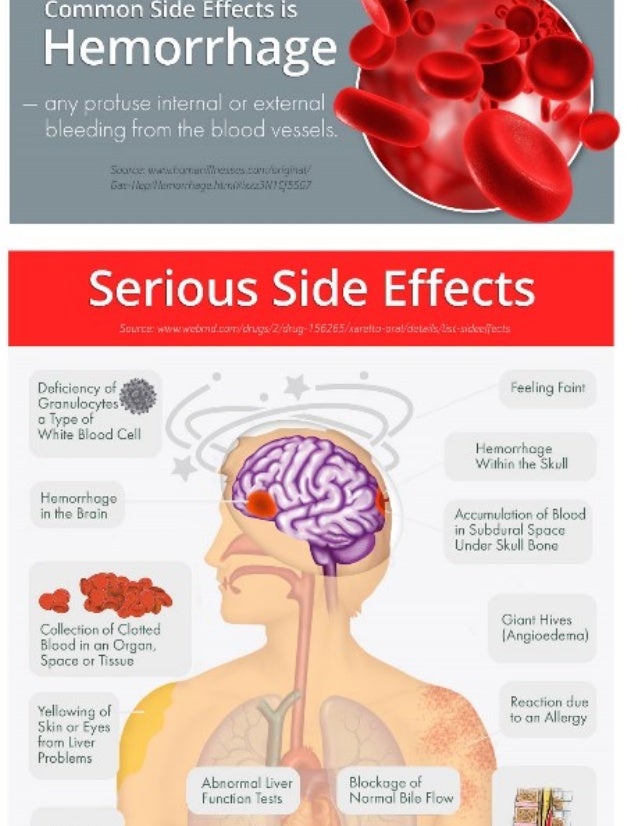
As with any anticoagulant the most serious adverse effect is bleeding, including severe internal bleeding. There is currently no antidote for rivaroxaban (unlike warfarin, the action of which can be reversed with vitamin K or prothrombin complex concentrate), meaning that seriously bleeding may be difficult to manage. A possible antidote (andexanet alfa) is being investigated.
Mechanism of action
Rivaroxaban is an oxazolidinone derivative optimized for inhibiting both free Factor Xa and Factor Xa bound in the prothrombinase complex. It is a highly selective direct Factor Xa inhibitor with oral bioavailability and rapid onset of action. Inhibition of Factor Xa interrupts the intrinsic and extrinsic pathway of the blood coagulation cascade, inhibiting both thrombin formation and development of thrombi. Rivaroxaban does not inhibit thrombin (activated Factor II), and no effects on platelets have been demonstrated.
Rivaroxaban has predictable pharmacokinetics across a wide spectrum of patients (age, gender, weight, race) and has a flat dose response across an eightfold dose range (5-40 mg). Clinical trial data have shown that it allows predictable anticoagulation with no need for dose adjustments and routine coagulation monitoring. However, these trials have excluded people with significant liver disease and end-stage kidney disease; therefore, the safety of rivaroxaban in these populations is unknown.

Chemistry
Rivaroxaban bears a striking structural similarity to the antibiotic linezolid: both drugs share the same oxazolidinone-derived core structure. Accordingly, rivaroxaban was studied for any possible antimicrobial effects and for the possibility of mitochondrial toxicity, which is a known complication of long-term linezolid use. Studies found that neither rivaroxaban nor its metabolites have any antibiotic effect against Gram-positive bacteria. As for mitochondrial toxicity, in vitro studies found the risk to be low.
Related drugs
A number of anticoagulants inhibit the activity of Factor Xa. Unfractionated heparin (UFH), low molecular weight heparin (LMWH), and fondaparinux inhibit the activity of factor Xa indirectly by binding to circulating antithrombin (AT III). These agents must be injected. Warfarin, phenprocoumon, and acenocoumarol are orally active vitamin K antagonists (VKA) which decrease the liver's production of a number of coagulation factors, including Factor X. In recent years, a new series of oral, direct acting inhibitors of Factor Xa have entered clinical development. These include rivaroxaban, apixaban, betrixaban, LY517717, darexaban (YM150), and edoxaban (DU-176b).

Research
In October 2014, Portola Pharmaceuticals announced the completion of Phase I and Phase II clinical trials for andexanet alfa as an antidote for Factor Xa inhibitors with few adverse effects, as well as the start of Phase III trials. The compound has yet to be approved by the U.S. Food and Drug Administration.
Are You Looking for Products
Here some products related to "Rivaroxaban".
Equate - Motion Sickness ..
Olympian Labs Biocell Col..
Decals - Decals & Bumper ..
Decals - Decals & Bumper ..
Get these at Amazon.com* amzn.to is official short URL for Amazon.com, provided by Bitly
Source of the article : here






EmoticonEmoticon